Transparent conducting oxide (TCO) thin films are widely used in flat panel displays, light emitting diodes and thin-film solar cells. In order to develop the technologies more efficient and less expensive, there is great demand for new improved TCO materials. An important task is to develop and produce indium free TCO films due to the high price and toxicity of indium. Aluminium doped zinc oxide (AZO) is considered as a potential alternative to the most commonly used indium tin oxide (ITO). Although AZO is extensively studied and used as a TCO material, there is still a lack of understanding of the relationship between native and impurity type of defects and deposition conditions and the effect on optical and electrical properties of the films. In order to be able to use TCO films in transparent electronics, it is necessary to produce a high quality and stable p-type TCO material, since the most TCOs are n-type. In addition, deposition process of the TCO films should be suitable for integrating TCO into multi-layer structures and large-scale production.
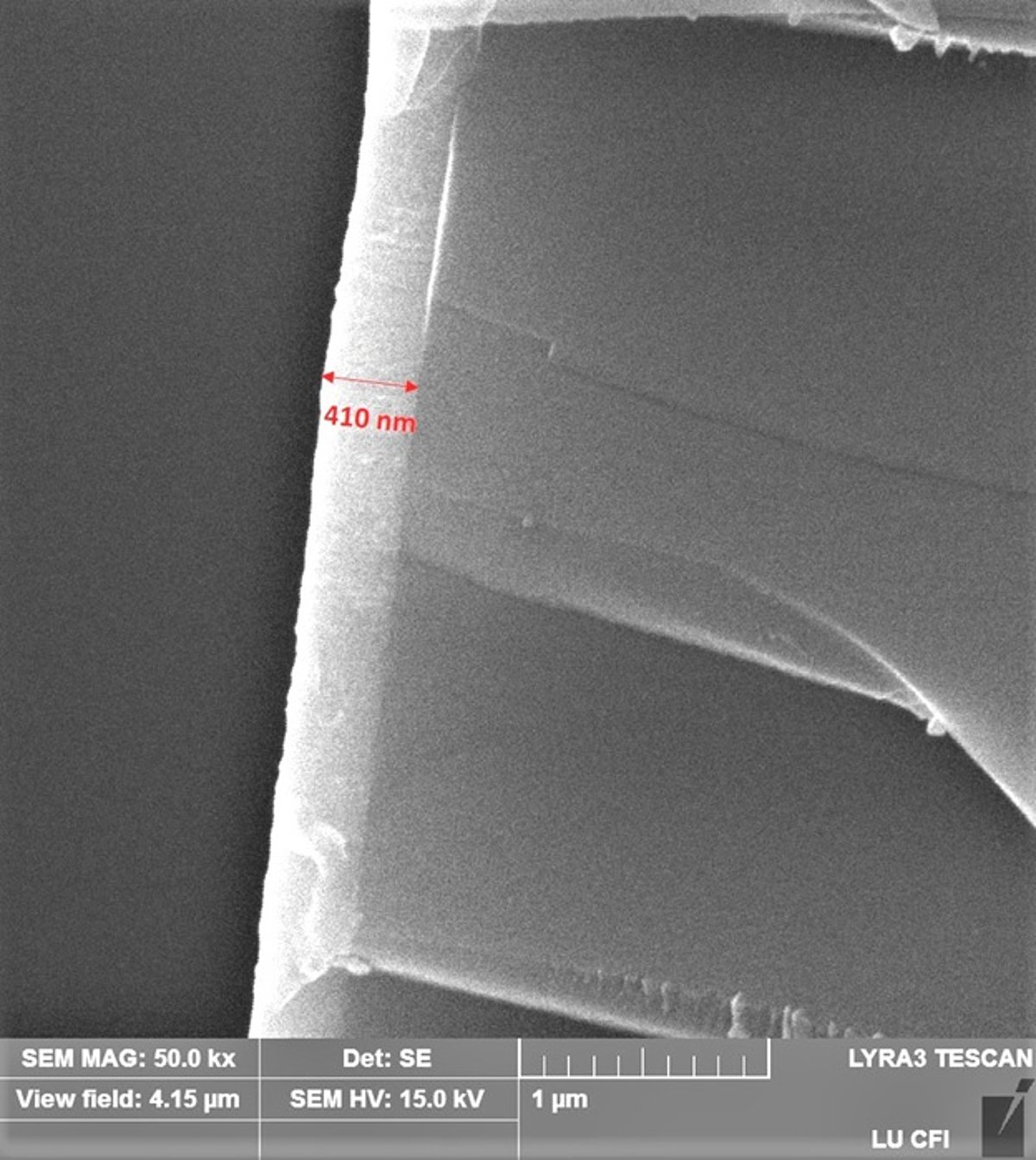
In this study, the n-type AZO and the p-type ZnO:Ir and Zn-Ir-O thin films have been deposited by reactive DC magnetron sputtering technique which is suitable for deposition on large-area substrates. The films were characterized by XRF, XRD, XAS, SEM, Raman and FTIR spectroscopy, electrical conductivity, Hall effect, thermoelectric measurements and visible light transmittance and reflectance. The properties of the films were analysed depending on the deposition parameters and composition.
During the study, the deposition process of AZO films has been developed. The influence of oxygen flow rate on the structure and properties of the AZO films has been established. The study demonstrates and explains the rapid change in the ratio of zinc and aluminium concentrations at low oxygen flow. Low resistivity (10-4 Ωcm) is obtained in a narrow oxygen flow range. The electrical activation of Al impurities and the structure of the films rapidly changes depending on the oxygen flow used in the reactive process. Due to this effect a rapid change in free electron concentration and mobility occurs.
ZnO doping with iridium results in degradation of the crystalline structure until the structure becomes completely amorphous in the Ir concentration range between 7 and 16 at.%. As a result of the change in Ir concentration, Ir ion charge state transition between 5+ and 4+ is observed. A new and intense band around 720 cm-1 has been detected in the Raman spectra of the ZnO:Ir and Zn-Ir-O thin films and may be attributed to the stretching mode of the O2-2 peroxide ions. By increasing the Ir concentration, there is a rapid increase in electrical conductivity and visible light absorption. In the Ir concentration range from 12.4 to 16.4 at.% there is a transition from the n-type to p-type conductivity.

 Academic Centre
Academic Centre